P5
Effects of the Alarmin S100A8/A9 on the Platelet and Neutrophil Response during Pulmonary Inflammation
Summary
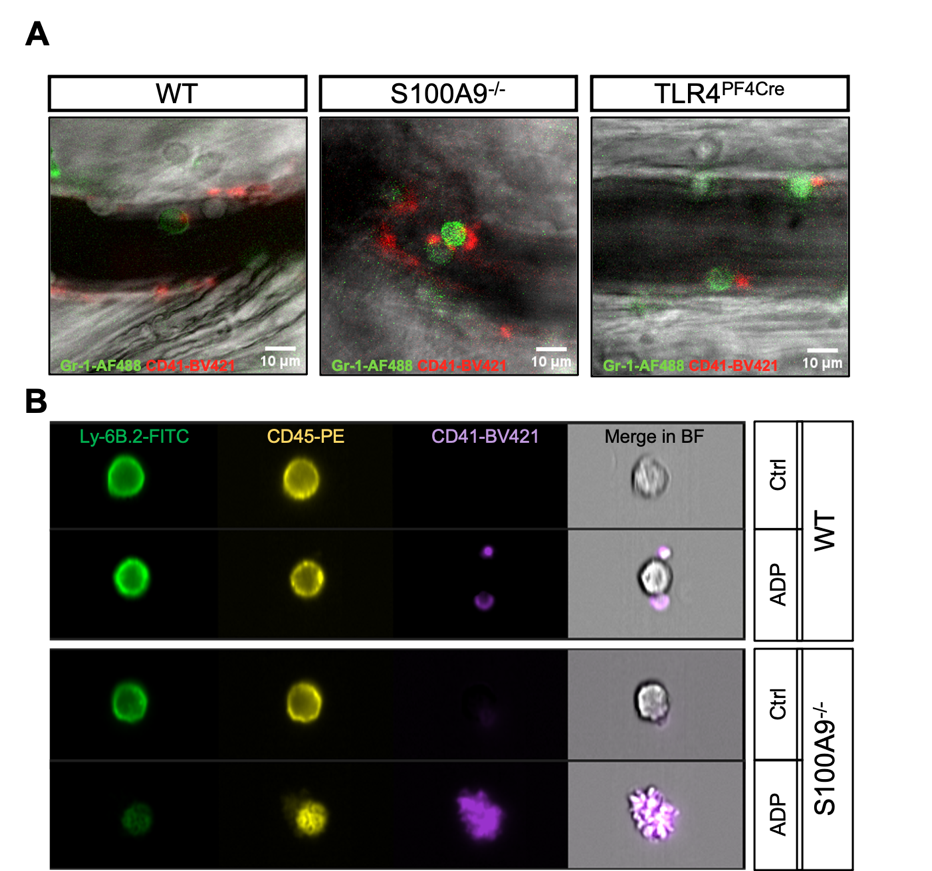
Figure: A) Platelet-neutrophil complexes in postcapillary venules of the murine cremaster muscle and B) neutrophil-platelet complexes in the circulation.
The endothelial glycocalyx is a dynamic structure localized at the luminal side of the endothelium and plays a central role in the context of vascular permeability. Main components of the glycocalyx are membrane-bound proteoglycans and glycoproteins incorporating plasma- and endothelium-derived soluble components and is part of the endothelial barrier. Vascular inflammation leads to the degradation of the endothelial glycocalyx which is related to altered vascular permeability. How neutrophil-derived S100A8/A9 affects NET formation and subsequently modulates the activation and participation of platelets in the leukocyte recruitment, the degradation of the glycocalyx, and the expansion and/or activation of myeloid-derived suppressor cells (MDSCs) is unknown and will be the subject for investigation within this project proposal.



