P8
The renin-angiotensin II axis: a novel target for the treatment of acute kidney injury
Summary
The development of AKI significantly complicates the clinical management of patients with systemic inflammation and is associated with unfavorable health outcomes. Recent epidemiological studies have shown that even mild or short episodes of AKI in sepsis survivors predispose for a greater risk of developing chronic and end-stage kidney disease in later life. Attempts to prevent AKI have largely been futile so far. Prior studies often started with the interventions after an AKI event, when a decline of kidney function (i.e., glomerular filtration rate) was already present. Application of norepinephrine is currently considered as the first-line therapy for the treatment of vasoplegia, but all catecholamines have adverse effects, including myocardial ischemia and arrhythmias. In a recent observational trial, we demonstrated that there is a dysregulation in the renin-angiotensin-aldosterone system (RAAS) likely caused by a reduced angiotensin-converting enzyme (ACE) activity after cardiac surgery.
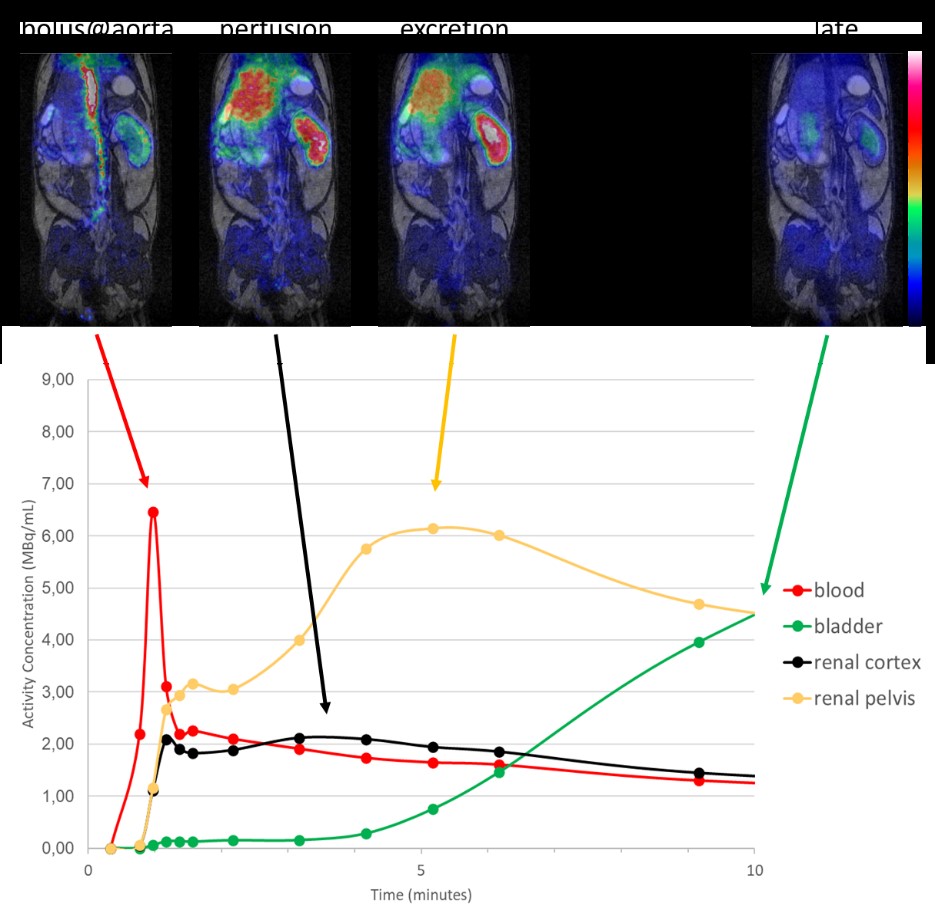
Figure: Dynamic whole-body [18F]-FDG-PET-MRI in mice
In this project we investigate if the administration angiotensin II ameliorates the AKI pathogenesis by modulating both the regulation of the microhemodynamics and the glomerular filtration as well as the inflammatory response in the kidney. Multiscale and translational imaging approaches ranging from intravital microscopy to functional/metabolic and AT1 receptor whole-body imaging will be established and applied to correlate regional kidney perfusion, function and inflammation, and AT receptor density during the spontaneous course of the disease and following angiotensin II-targeted interventions.


